Eli Lilly Suspends COVID-19 Antibody Therapy Trial Over “Potential Safety Concern”
 By Tyler Durden
By Tyler Durden
Barely a week after applying for emergency use approval from the FDA for one of its COVID-19 antibody therapeutics, it appears trials for one of Eli Lilly’s therapeutic drugs focused on the virus (Eli Lilly is working on more than just one) have been paused.
News of the trial was broken by a doctor on Twitter before it was swiftly confirmed by several media organizations.
<BREAKING NEWS>https://t.co/UBH7CzwLj7 has learned that the ACTIV-3 trial testing Eli Lilly's monoclonal antibody LY-CoV555 has been PAUSED.
Trial tests the antibody + remdesivir vs remdesivir alone.
President Trump received similar treatment.
Brief: https://t.co/Dmwe7qqN1v pic.twitter.com/sqUBICRXxa
— Brief19, your daily roundup of SARS-CoV-2. (@Brief_19) October 13, 2020
[do_widget id=text-16]
CNBC confirmed that the trial that was paused is for the antibody treatment for which Eli Lilly applied for the EUA, which is probably why the market has reacted so negatively.
Lilly confirms @jeremyfaust report that one of the trials evaluating its antibody drug for #covid19 has paused enrollment pic.twitter.com/RaRhM75B0t
— Meg Tirrell (@megtirrell) October 13, 2020
Per the NYT report, the government-sponsored clinical trial has been paused because of a “potential safety concern.” The report cited emails that government officials sent on Tuesday to researchers at testing sites, which the company confirmed. The trial was designed to test the benefits of the therapy on hundreds of people already infected with the virus. The company did not say how many volunteers were sick, or any details about their illness.
Eli said in a statement that the independent monitoring board recommended the pause (unlike the JNJ pause announced last night, which was undertaken voluntarily, according to JNJ).
The pause in enrollment comes after Johnson & Johnson confirmed that it had voluntarily paused its Phase 3 vaccine trials after a participant in the 60k person trial came down with an unusual and unspecified illness. An AstraZeneca trial in the US has still not restarted more than a month after issues with a couple of patients caused them to briefly pause all trials around the world, though all the others have restarted.
ELI LILLY ANTIBODY TRIAL IS PAUSED BECAUSE OF POTENTIAL SAFETY- NYT REPORTER pic.twitter.com/w70hWf8D5I
— Quoth the Raven (@QTRResearch) October 13, 2020
As we noted last night, convincing the public to trust the vaccines is critically important, according to a team of analysts at Goldman Sachs, who recently warned that convincing the public to accept the vaccine is an understated risk.
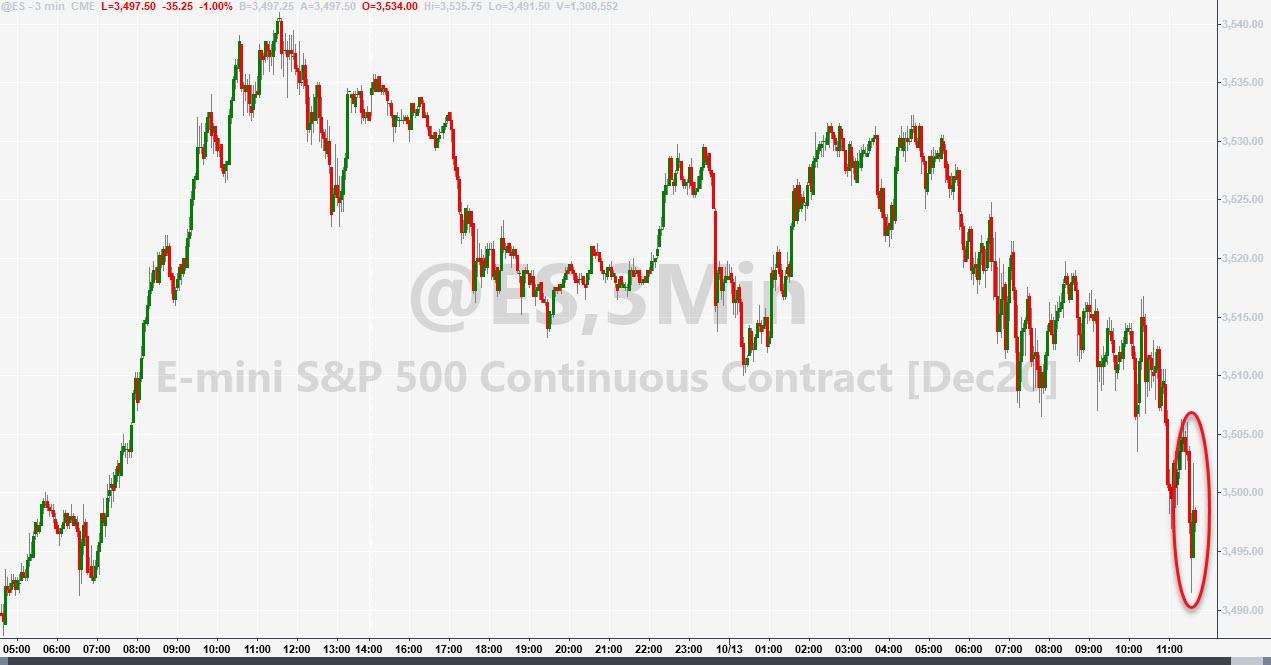
Stocks tumbled on the news, which is hardly a surprise given that President Trump and even Dr. Fauci have hailed these types of therapeutic as critical in saving lives.
Image: Pixabay
FREE PDF: 10 Best Books To Survive Food Shortages & Famines